Light Shed on Complex Dynamics of How Organisms Sense Infection
Complete the form below to unlock access to ALL audio articles.
Included in the vast fallout stemming from the COVID-19 pandemic, scientists are paying closer attention to microbial infections and how life forms defend against attacks from pathogens.
Research led by University of California San Diego scientists has shed new light on the complex dynamics involved in how organisms sense that an infection is taking place.
UC San Diego Assistant Project Scientist Eillen Tecle in Professor Emily Troemel's laboratory (Division of Biological Sciences) led research focusing on how cells that are not part of the conventional immune system respond to infections when pathogens attack. Scientists have conducted extensive research on so-called "professional" immune cells that are defensive specialists. Much less is known about how "non-professional" cells handle such threats.
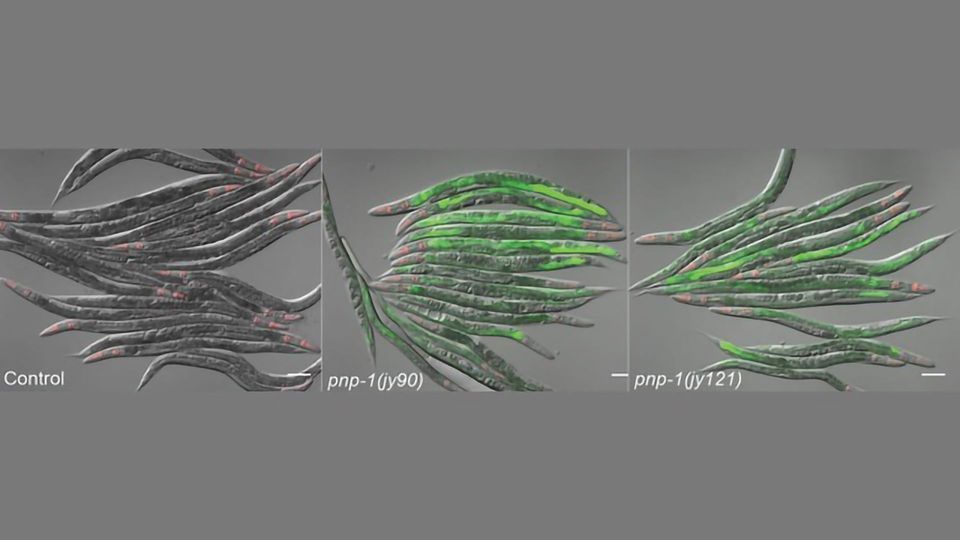
Tecle, Troemel and their colleagues at Pennsylvania State University focused their research on roundworms (Caenorhabditis elegans), animals that lack dedicated immune cells, to help decipher details of such dynamics.
As described in the journal PLOS Pathogens, the researchers conducted experiments involving roundworms under attack by viruses and microsporidia, which are natural pathogens of worms and humans. The results indicate that roundworms may sense changes in their metabolism in order to unleash protective defenses, even if they don't directly sense the pathogen incursion.
In their study, the researchers examined how hosts may respond when pathogens such as viruses and microsporidia steal key compounds from C. elegans cells known as nucleotides. Pathogens like these are required to pilfer such components from their hosts in order to survive. The study's results focused on biological pathways related to the breakdown of chemical compounds known as purine nucleotides. This purine metabolism pathway is key to the cells' ability to sense alterations as a way to induce an immune response.
"We hypothesize that the host has ways to surveil what's going on inside of its cells in an active process," said Tecle. "Our results suggest that the host has developed ways to sense the theft of purine metabolites. It seems that when these key cellular building blocks are stolen by the pathogen, the host senses this theft to mount an immune response to the pathogen."
This research may shed light on why purine-related compound mutations have been found to underlie many human diseases, including adenosine deaminase deficiency, which damages the immune system, and Lesch-Nyhan syndrome, which involves neurological and behavioral abnormalities. While these mutations result in various disorders in humans, they may persist in the human population to provide some protection against infections, for example during viral pandemics.
"Particularly in the context of the COVID-19 pandemic, it's so important that we continue to study these questions of immunity in lots of different systems to build new tools so that we can learn how to prevent and treat infections," said Troemel.
Reference: Tecle E, Chhan CB, Franklin L, et al. The purine nucleoside phosphorylase pnp-1 regulates epithelial cell resistance to infection in C. elegans. PLOS Pathogens. 2021;17(4):e1009350. doi: 10.1371/journal.ppat.1009350
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source.



