Using Spectroscopy to Diagnose Skin Cancer

Complete the form below to unlock access to ALL audio articles.
Optical methods have been very widely investigated for initial diagnostics of skin pathologies in the last few decades. Easy access to skin tissues and a high incidence of skin tumors, as well as the superior sensitivity of optical detectors and novel imaging approaches, make this a very attractive area of research for new diagnostic methods.
Our work focuses on light-induced autofluorescence spectroscopy (LIAFS) and diffuse reflectance spectroscopy (DRS), which offer complementary information and can be combined very successfully. LIAFS is useful for detecting biochemical changes occurring in skin disorders, while DRS reveals morphological changes and the presence of diagnostically important skin pigments. Combining the two increases the total diagnostic accuracy compared with using either technique alone.
We carried out in vivo investigations as part of a clinical trial of a spectral diagnostic system for skin cancer detection at the Tsaritsa Yoanna – ISUL University Hospital, in Bulgaria.5 Lesions were initially classified using a dermatoscope and ABCD (asymmetry, border irregularity, color unevenness, diameter) scoring criteria. The second step was to measure LIAFS spectra of the lesion and surrounding normal skin using excitation at 365, 385, and 405nm from narrow-band LEDs. We obtained DRS data using a broadband (400–900nm) halogen lamp. Every lesion also received a ‘gold-standard’ histological examination. We recorded and stored spectral data using a fiber-optic microspectrometer USB4000 with SpectraSuite software (Ocean Optics Inc., Dunedin, FL). Normal tissue spectra detected were used as a basis for comparison with the pathologies observed. Spectra have been obtained from more than 600 patients to date. All patients were Caucasians with skin phototypes II and III, which are the most common skin types in Bulgaria. Skin pigmentation (melanin levels) will influence the spectra. Melanin levels also affect rates of sun-related neoplasia, which are very uncommon in people with phototypes IV (Latin skin tones) and V (Arab and Asian skin tones).
We recorded LIAFS and DRS in vivo for several groups of cutaneous pathologies: benign, dysplastic, and malignant lesions. Malignant lesions are divided between melanin-pigmented malignant melanoma (MM, 41 cases) and non-melanoma skin tumors, including basal cell carcinoma (BCC, 141 cases), squamous cell carcinoma (SCC, 28 cases), and keratoacanthoma (12 cases). Figure 1 shows LIAFS and DRS spectra averaged from sets of patients with normal skin, BCC, SCC, and MM.

Figure 1. (a) Light-induced autofluorescence spectroscopy (LIAFS) measurements using excitation at 405nm and (b) diffuse reflectance spectroscopy (DRS) measurements at 400–900nm for the most common cutaneous malignancies: basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and malignant melanoma (MM), averaged by set of patients with these diagnoses, proved by their histology. a.u.: Arbitrary units.
The LIAFS spectrum detected in vivo is a superposition of fluorescence spectra of endogenous fluorophores existing in the tissue, distorted by partial re-absorption by tissue pigments (mainly blood and melanin).6, 7 The endogenous fluorophores detected are some structural proteins (such as collagen, elastin, and keratin), protein cross-links, and co-enzymes (nicotinamide adenine dinucleotide, or NADH, and flavins). In advanced tumors, red-fluorescent porphyrins are also observed. DRS spectra obtained from identical anatomical sites from different patients have similar features, with minima at 400–420, 543, and 575nm related to hemoglobin absorption. Exponentially decreasing melanin absorption from UV to near-IR reduces the signal at shorter wavelengths.
We found that for each given pathology the spectral shapes and intensity showed similar behavior across patients and we observed trends, objective and repeatable from patient to patient, for given pathological changes. For example, BCC lesions always reveal fluorescence intensities lower than surrounding normal skin tissues, while SCC usually has fluorescence brighter than surrounding skin. This observation could be used to distinguish between these two kinds of non-melanoma malignancies.8 Malignant melanoma lesions have no significant spectral shape changes related to appearance of new fluorophores in their cells but always have extremely weak fluorescence, and we could distinguish them from dysplastic nevi by the level of the LIAFS signal with relatively moderate diagnostic accuracy (about 70%). We could improve this to more than 90% by also evaluating reflectance spectral features of the lesions.4
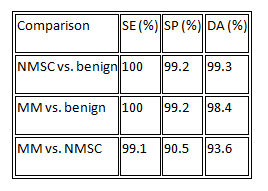
Applying combined discrimination algorithms to LIAFS and DRS spectral features enabled us to improve the sensitivity, specificity, and accuracy of skin cancer diagnosis for normal skin, BCC, SCC, and MM: see Table 1. Based on the spectral differences observed for the major types of skin lesions (malignant, dysplastic, and benign), we extracted about 30 spectral parameters, such as intensity levels, specific minima and maxima, intensity ratios, and slopes of the spectra in a given range, that we used to develop a multispectral discrimination algorithm to differentiate lesions by specific clinical needs.
Table 1.Statistical data for LIAFS and DRS techniques to distinguish non-melanoma skin cancer (NMSC), which includes BCC and SCC, malignant melanoma, and benign lesions. SE: Sensitivity. SP: Specificity. DA: Diagnostic accuracy.
In summary, we have shown that combining the optical techniques LIAFS and DRS offers a good way to improve diagnostic accuracy by using complementary information about normal and diseased tissues. We correlated the presence and intensity of known fluorophores with spectral features and changes in the architecture and pigmentation of the lesion to make significant progress in diagnosing skin alterations in vivo. Our combined LIAFS and DRS approach is able to detect the changes that appear in cutaneous non-melanoma and melanoma tumors and to differentiate accurately dysplastic and benign pathologies. We are now planning to optimize the experimental system used for the LIAFS and DRS of skin neoplasia to develop it into an integrated device that is ‘user-friendly’ for clinicians and their patients.
This work is supported by the National Science Fund of Bulgaria under grant DMU-03-46/2011 ‘Development and introduction of optical biopsy for early diagnostics of malignant tumors.’ Ekaterina Borisova holds a 2014 Bulgarian UNESCO/L'Oréal co-sponsored personal Fellowship for Young Women in Life Sciences.



