When Art Meets Science and Food Coloring, Bioprinting Prospers

Complete the form below to unlock access to ALL audio articles.
A new open-source method for bioprinting represents a breakthrough for the field of regenerative medicine, and its success stems from a special ingredient: food dye.
This week’s cover of Science features a spectacular entangled network of blood vessel-like hydrogels, which is one of several constructs created by a team of bioengineers from Rice University, University of Washington, Duke University, Rowan University, and Nervous System (a design firm in Somerville, Massachusetts).
The group overcame key hurdles in bioengineering which has unlocked crucial design freedoms in the bioprinting world, evident in the other achievements reported today in Science. The method has been released as an open-source resource and enabled the development of a bioinspired alveolar model, a 3D functional bicuspid venous valve, and a carrier for liver tissue that was engrafted into a mouse model of chronic liver injury.
Overcoming one of the biggest regenerative medicine roadblocks
As the number of people waiting for organ transplants greatly exceeds the number of organs available, alternative solutions are needed. Therefore, significant interest lies in the field of regenerative medicine and bioprinting, which could theoretically allow replacement organs to be printed from a patient’s own cells, reducing the risk of organ rejection.Kelly Stevens, co-author and assistant professor in the Departments of Bioengineering and Pathology at the University of Washington believes this theoretical solution will become a reality:
"We envision bioprinting becoming a major component of medicine within the next two decades."
One of the biggest challenges to generating functional tissue replacements has been the inability to deliver oxygen and nutrients to all cells in an artificial organ or tissue transplant. Without blood vessels to supply nutrients and remove waste, tissues will not survive for very long. Therefore, the team sought a method for printing intricate vasculature that would allow tissue to thrive, explained Jordan Miller, coauthor and assistant professor in the Department of Bioengineering at Rice University:
“The general idea of our field is trying to understand the structure of human tissues. And I think that one of the things that our field has really been missing is some of that multivascular architecture that we have in the body. So if we hope to build anything that resembles the human lung, we don't need just one vessel network, we actually need at least two, because we need to have the airway and the bloodstream.”
Food coloring shapes regenerative medicine
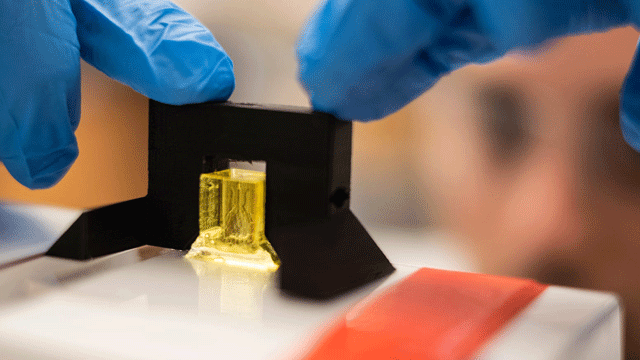
To build the soft vessels, a form of 3D printing was used called “stereolithography” which relies on light to cause monomers to link together. This process can be tightly controlled; while the xy resolution is determined by the light path, the z resolution is dictated by additives that absorb excess light and restrict the polymerization to the desired layer thickness.However, finding the right light-blocking additives wasn’t easy. This method is used in the fabrication of plastic parts, but the additives contain known genotoxic and carcinogenic properties – certainly not appropriate for biomanufacturing! Therefore, the team hunted for nontoxic light blockers. They tested a few promising organic candidates: yellow food coloring, curcumin (from turmeric) and anthocyanin (from blueberries), as well as inorganic gold nanoparticles (50 nm) which are known for their biocompatibility and light-attenuating properties.
 Rice University bioengineer Daniel Sazer prepares a scale-model of a lung-mimicking air sac for testing. In experiments, air is pumped into the sac in a pattern that mimics breathing while blood is flowed through a surrounding network of blood vessels to oxygenate human red blood cells. (Image credit: Jeff Fitlow/Rice University)
Rice University bioengineer Daniel Sazer prepares a scale-model of a lung-mimicking air sac for testing. In experiments, air is pumped into the sac in a pattern that mimics breathing while blood is flowed through a surrounding network of blood vessels to oxygenate human red blood cells. (Image credit: Jeff Fitlow/Rice University)For these studies, the yellow food dye (tartrazine) was selected as the winner of the photoabsorber competition. Miller commented on what that meant for the project:
“With this innovation in using these food dyes as light-blockers, we have so much new vascular design freedom. We’re able to make lots of different things in these soft hydrogels that are based on water that we have not been able to make before.”
One giant leap for biomaterials tissue engineering
Thanks to the newfound creative freedom, and the design expertise of Nervous System, a design studio that works at the intersection of science, art, and technology, the group crafted a synthetic vascular system made from transparent hydrogels. Furthermore, the vessels were incorporated around a functional mimic of lung tissue that was 3D printed using the same technique.The vessel/lung structure passed a few significant tests:
- The vessels were strong enough to withstand the “breathing” motion of the alveolar models
- The alveolar model delivered oxygen to red blood cells that passed through the vessels
- 3D bicuspid valves were incorporated that responded rapidly to changes in flow direction
Miller recalls one of the most exciting moments of the project:
“It was the first time that we started using the pneumatic system to ventilate the airway. It really did look like it was breathing… and it was so startling in the complexity of the architecture – we were seeing new things in the bloodstream immediately, as soon as we started doing that. It really transformed our view of what's possible inside of the soft materials that are more than 80% water.”
Video credit: Rice University
Looking ahead to replacement organs
To better mimic the biochemical conditions in vivo, the group showed that endothelial cells injected into the airway could line the vessels, and survive. Miller says that the group is looking to do similar types of experiments in blood vessel architectures.
“We have shown that human cells can survive the pre-hydrogel mixture, they can survive the polymerization process, and they survive inside the functional gel that we've made. One of the studies we put in the supplement was with mesenchymal stem cells. We were able to show that they're able to survive and grow and spread in 3D inside the gel. They were able to also differentiate towards a bone-like lineage of cells starting to prepare to mineralize that matrix. We really see the full potential of this technique is not just to model living tissue, but also to build it and understand its function in the body.”
As a test of the relevance of this approach to therapeutic implants, the team 3D printed hydrogel carriers, loaded them with hepatocyte aggregates, and implanted them into mice models of chronic liver injury. After 14 days of engraftment, there were positive signs; there were signs of surviving functional hepatocytes, and host blood was present in the explanted tissues.  Experiments performed by Rice University and University of Washington researchers explored whether hepatocytes would function normally if they were incorporated into a bioprinted implant and surgically implanted in mice for 14 days. (Image credit: Jordan Miller/Rice University).
Experiments performed by Rice University and University of Washington researchers explored whether hepatocytes would function normally if they were incorporated into a bioprinted implant and surgically implanted in mice for 14 days. (Image credit: Jordan Miller/Rice University).
Stevens shares her plans for the future: “Here, we tested just one liver cell function. But, liver cells have about 500 functions. In the future, we will test whether our bioprinted tissues can perform many more of these functions. We are also working to improve the resolution of our patterning, which is still about an order of magnitude larger than the size of most human cells.”
Science and art: a dream match
One key component to this work was the contribution from Nervous System, who Miller believes will help transform the field:“They have been writing their own software, to build architectures that are generative, based on algorithms that people observe in nature. And so my proposition to them a couple years ago was that we could use their algorithms to not only build structures that resemble living tissue, but that could actually be used to make living tissue.”
Jessica Rosenkrantz, co-founder and creative director of Nervous System (Twitter: @nervous_jessica, Instagram: @nervous_jessica) reflects on the opportunity:
“As designers whose work is inspired by the complex patterns we see in nature, it's a dream come true to be able to work on designing living things. In our studio, we develop generative software for design of complex geometries. These sort of tools are a perfect fit for tissue engineering where we need to create intricate, customized structures like vasculature.”

A U.S. one-cent coin shown next to a scale-model of a lung-mimicking air sac with airways and blood vessels. Image credit: Brandon Martin/Rice University
"We believe that science should be open-source"
This work represents significant progress for the field of bioengineering and regenerative medicine, and opens doors for work in microfluidics, organ-on-a-chip models of pathology, and drug discovery.Miller acknowledges the value of open-source technology, and is pleased to be able to give back to the community that helped drive this project:
“Another exciting part of this work is that all of our equipment that we used to both 3D print the vessel structures in the gel, and to do the ventilation - that's all been open-sourced. Later today people will be able to download it legally and freely. I think that’s going to allow other people to really explore this space in ways that we haven’t seen before.”
Edit: CELLINK, the 3D bioprinting company can be found here: https://cellink.com/about-us/
Reference:
Grigoryan, B., Paulsen, S., Corbett, D., Sazer, D., Fortin, C., Zaita, A., Greenfield, P., Calafat, N., Gounley, J., Anderson, H., Johansson, F., Randles, A., Rosenkrantz, J., Louis-Rosenberg, J., Galie., P., Stevens, K., Miller, J. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science doi: 10.1126/science.aav9750




