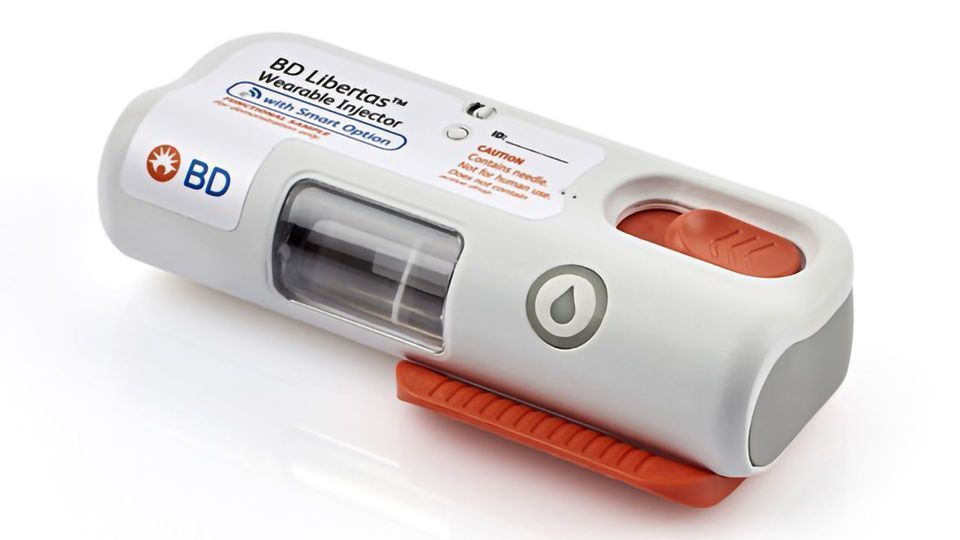
BD Completes Clinical Trial for BD Libertas™ Wearable Injector
Complete the form below to unlock access to ALL audio articles.
BD (Becton, Dickinson and Company), a global medical technology company, has announced the completion of a 50-subject human clinical trial with the BD Libertas™ Wearable Injector.
The injector is a subcutaneous drug delivery system, currently in development, that is designed to require no patient assembly and deliver biologics with viscosities up to 50 cP in 2-5 mL and 5-10 mL configurations.
The BD independently sponsored and conducted study was designed to evaluate the performance of the 5 mL BD Libertas device in human subjects, including tissue effects, skin reactivity and patient acceptance. The results are expected to be announced early 2020.
The study represents the most recent in a series of over 50 BD conducted pre-clinical and clinical studies intended to measure the performance of the BD Libertas™ Wearable Injector, demonstrate the feasibility of 2-10 mL biologic injections into subcutaneous tissue and characterize tissue response to large volume injections in human and animal subjects.



